Quick Facts: Terpene Vs. Terpenoid Structure
Author: Shauna Kelley | Posted: 06.14.2023
Most people in the cannabis industry have heard of terpenes, but have you heard of terpenoids?
Terpenes
Terpenes are naturally occurring compounds produced predominantly by plants for various reasons, such as defense and attraction. These compounds impact the aroma of plants, producing a strong odor which can protect the plant by deterring animals away. Terpenes are a large group of compounds called hydrocarbons, which are organic molecules that consist of carbon and hydrogen. Terpenes are made up of building blocks called isoprene units (C5H8). Building upon the isoprene unit creates the variation between terpenes. Monoterpenes are terpenes that contain two isoprene units (C10H16), and sesquiterpenes contain three isoprene units (C15H24).
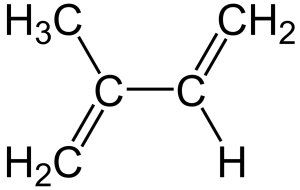 Structure of an isoprene unit (2-methylbuta-1,3-diene). Isoprene units can be linked together to form linear terpenes or can be formed into rings to form cyclic terpenes.
Structure of an isoprene unit (2-methylbuta-1,3-diene). Isoprene units can be linked together to form linear terpenes or can be formed into rings to form cyclic terpenes.
Terpenoids
Terpenoids are a large group of compounds derived from isoprene that have oxidized methyl groups. This means that oxygen has been added into the mix of carbon and hydrogens. Unlike terpenes, terpenoids contain various functional groups attached to the base. Functional groups are patterns of atoms that display a particular function or property. A common functional group attached to terpenoids is the hydroxyl group (-OH), classifying them as terpene alcohols. The hydroxyl group affects the compounds polarity, changing the physical properties of that compound.
Examples
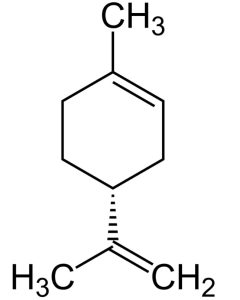
Limonene is a cyclic monoterpene
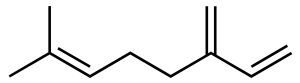 beta-Myrcene is a linear (acyclic) monoterpene
beta-Myrcene is a linear (acyclic) monoterpene
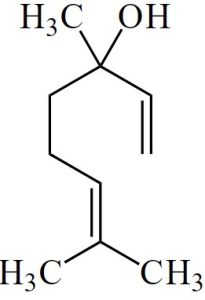
Linalool is a monoterpenoid, containing a hydroxyl group (-OH).
